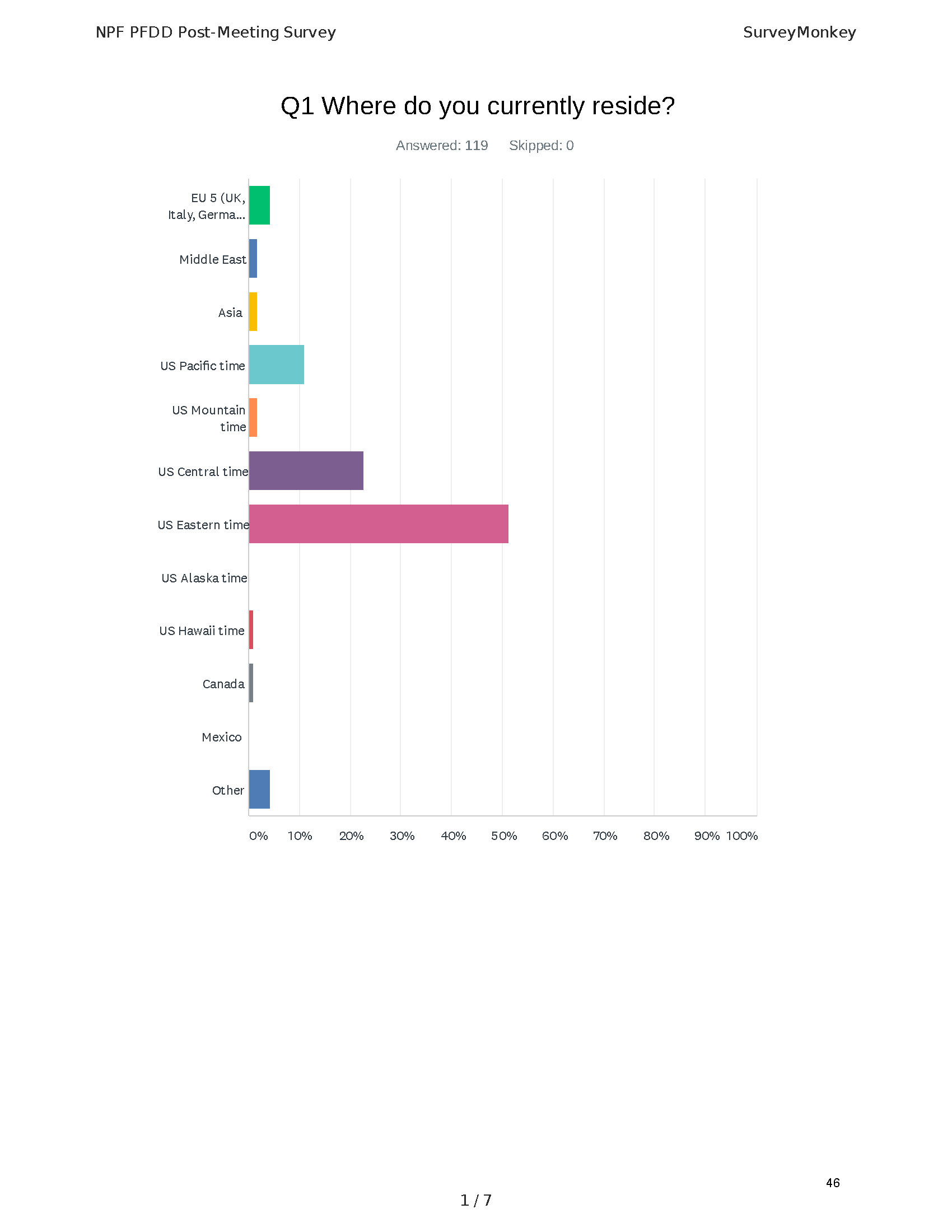
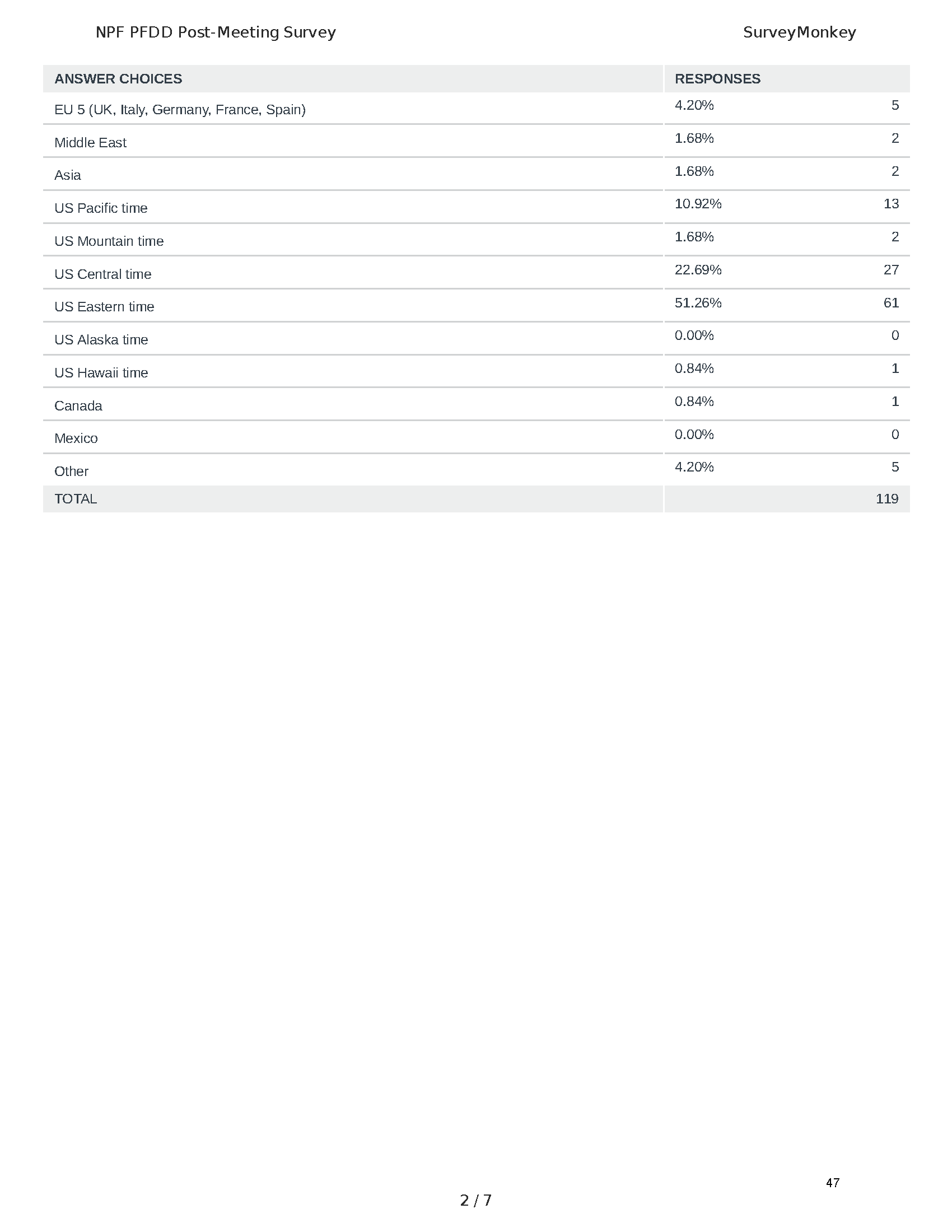
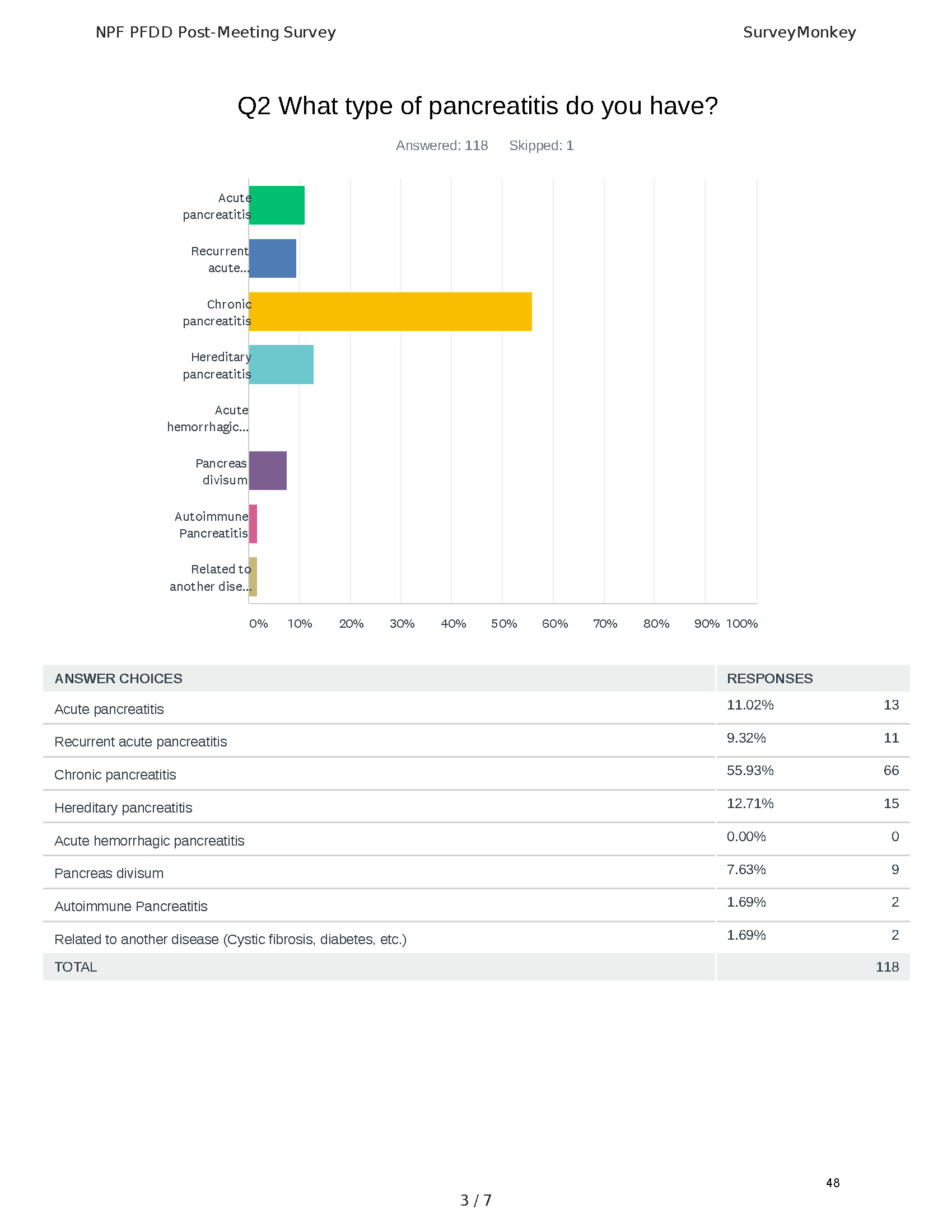
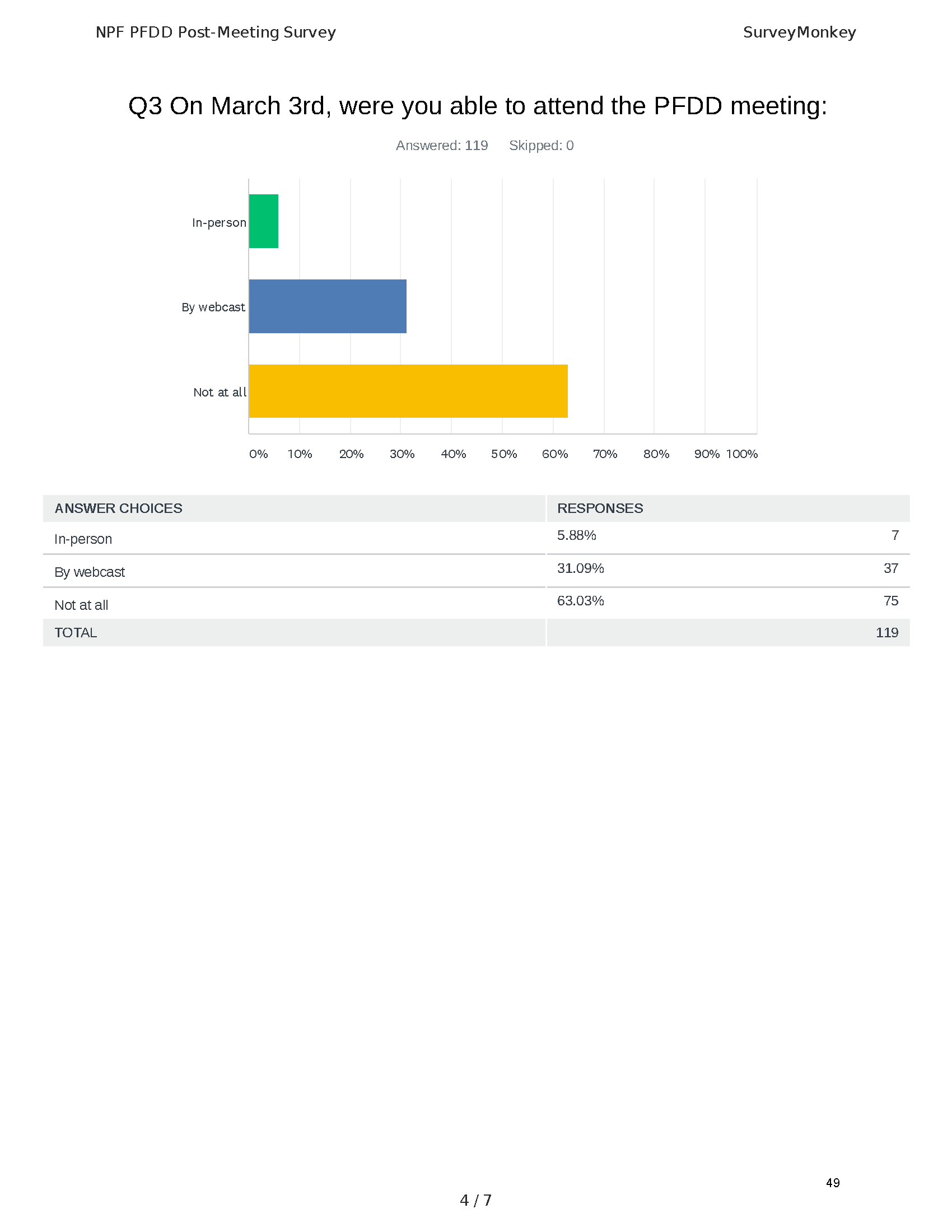
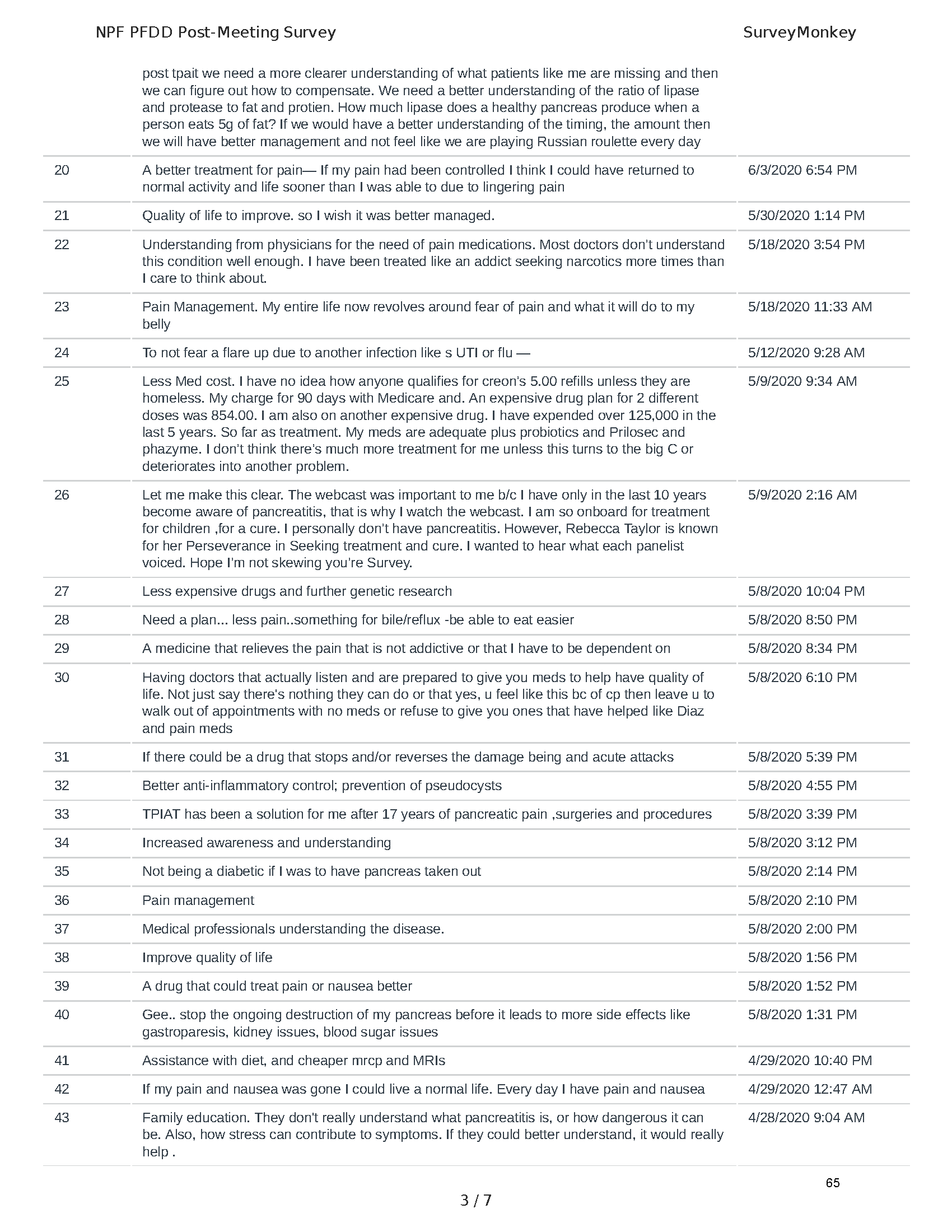
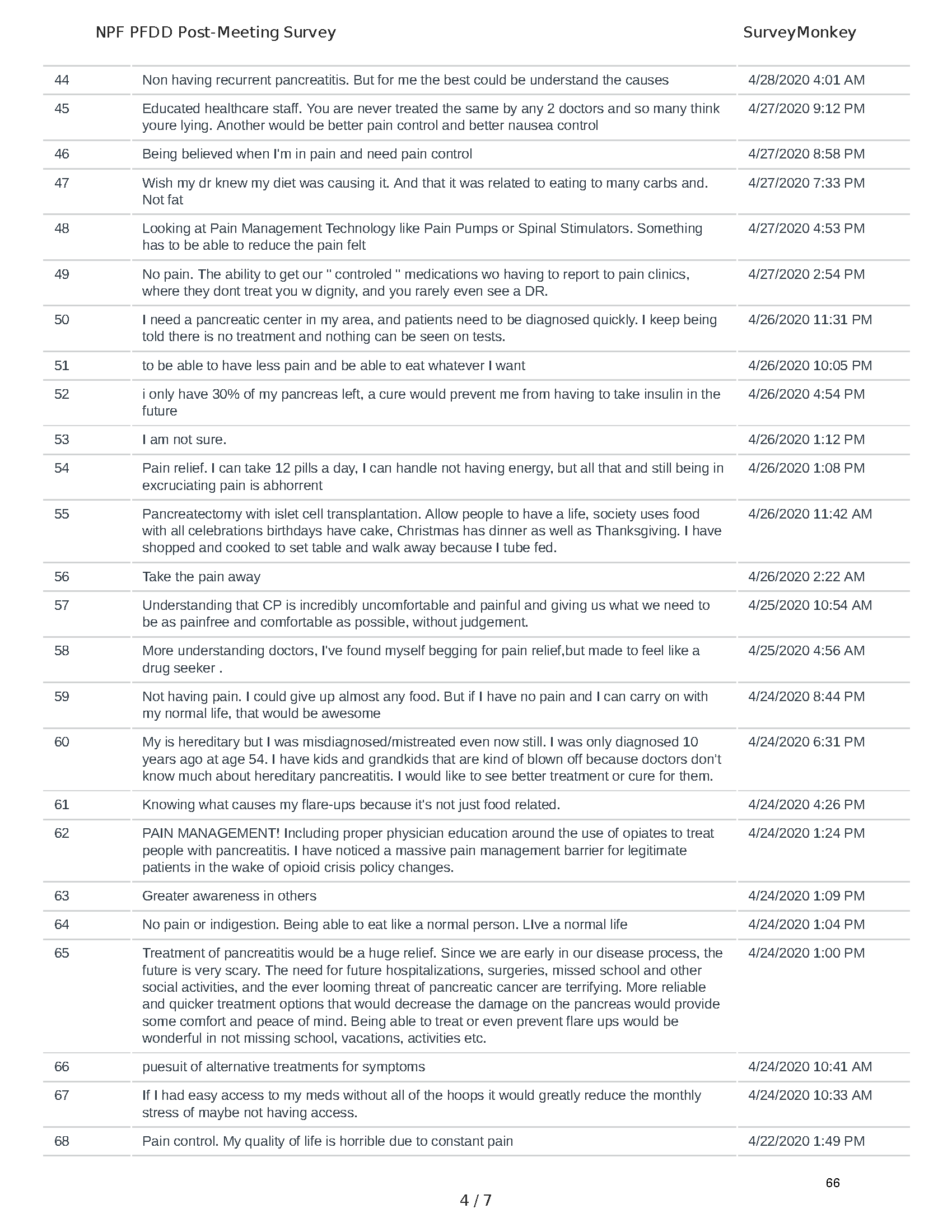
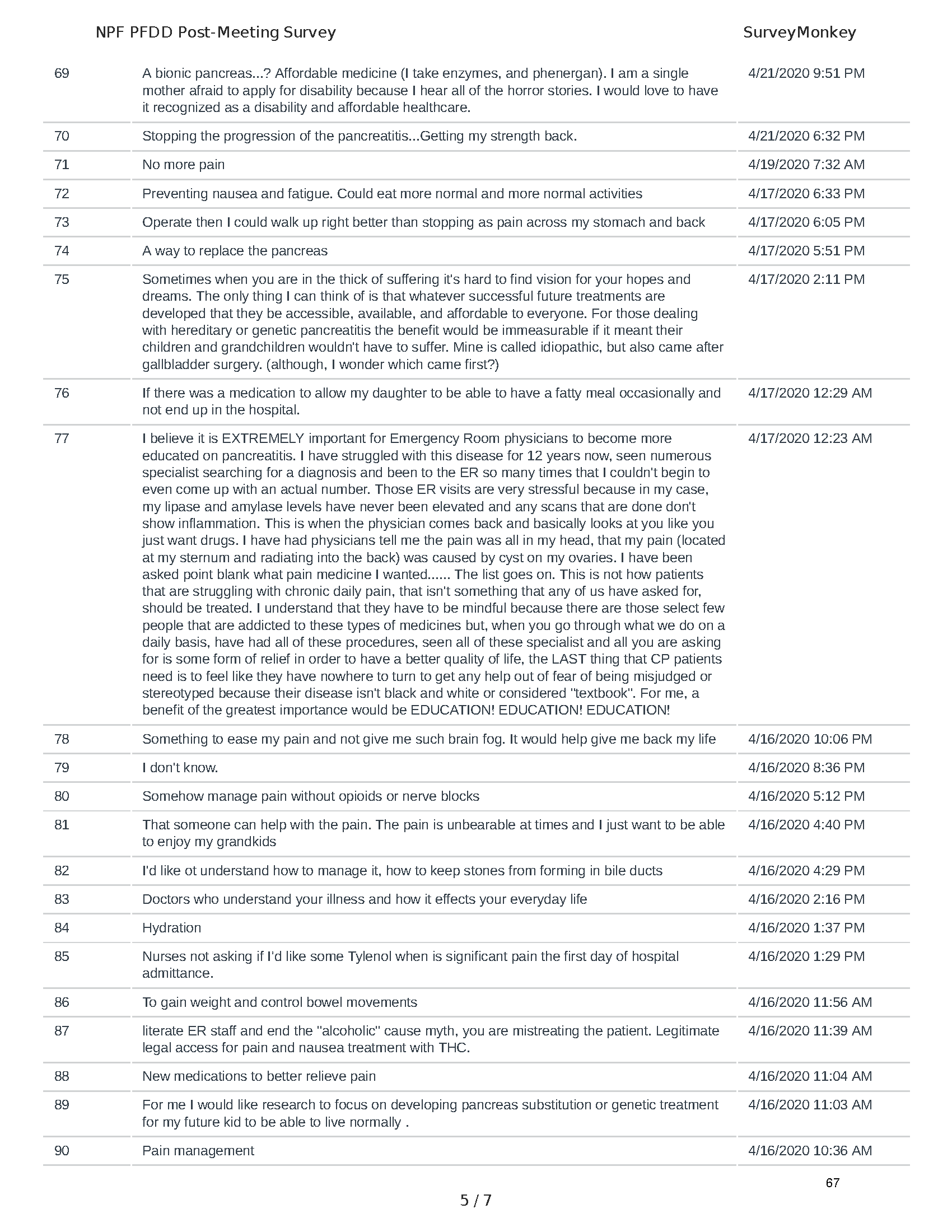
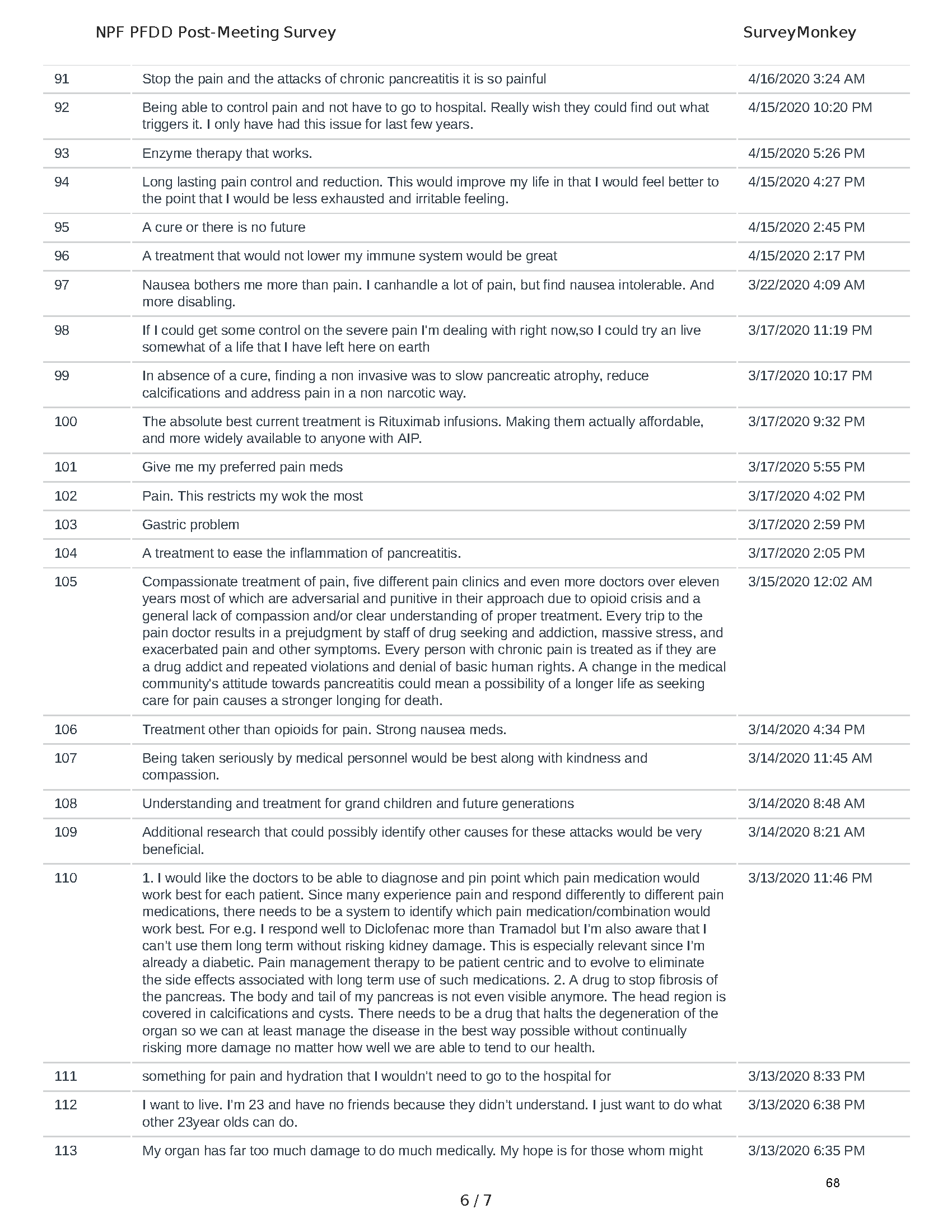
Pancreatitis Externally-Led Patient-Focused Drug Development Meeting
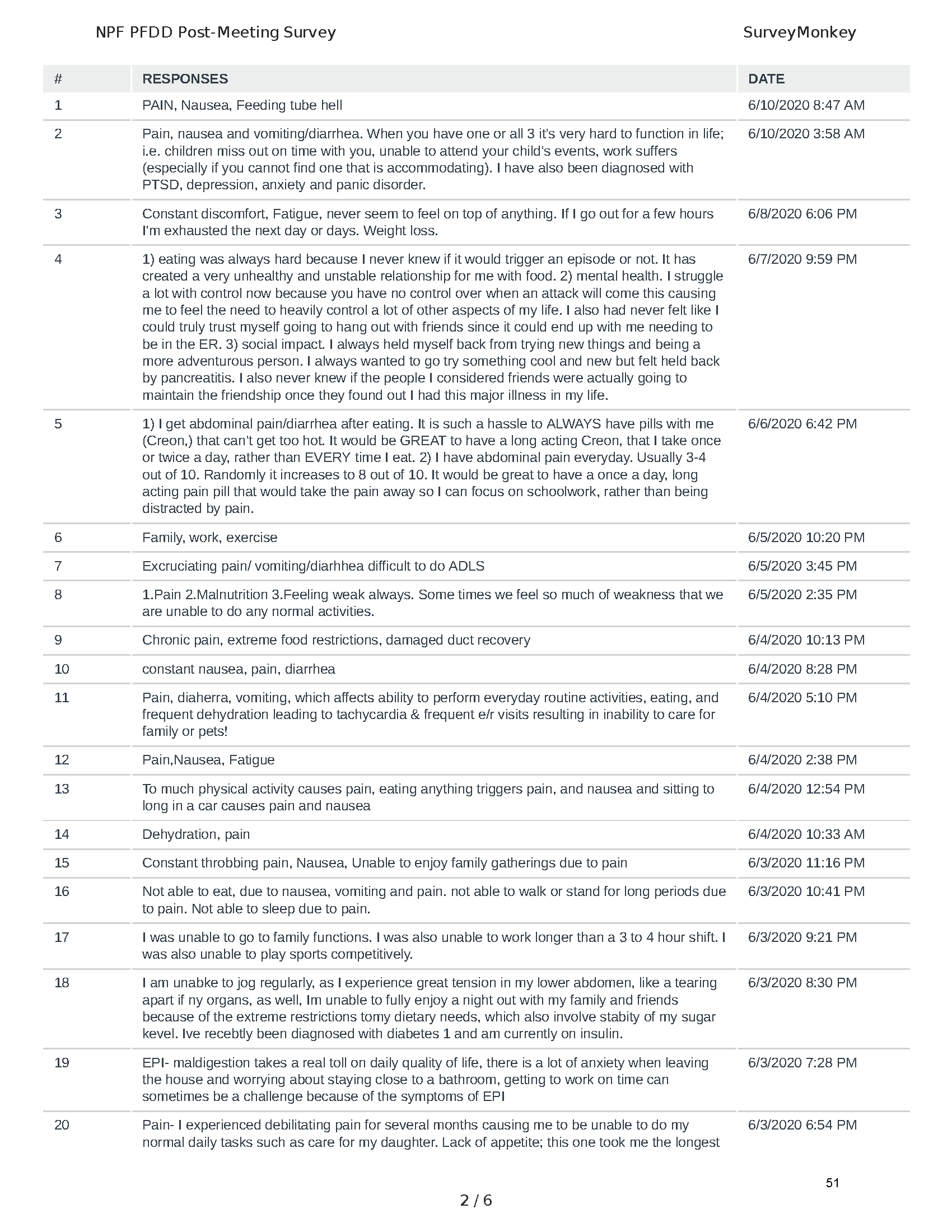
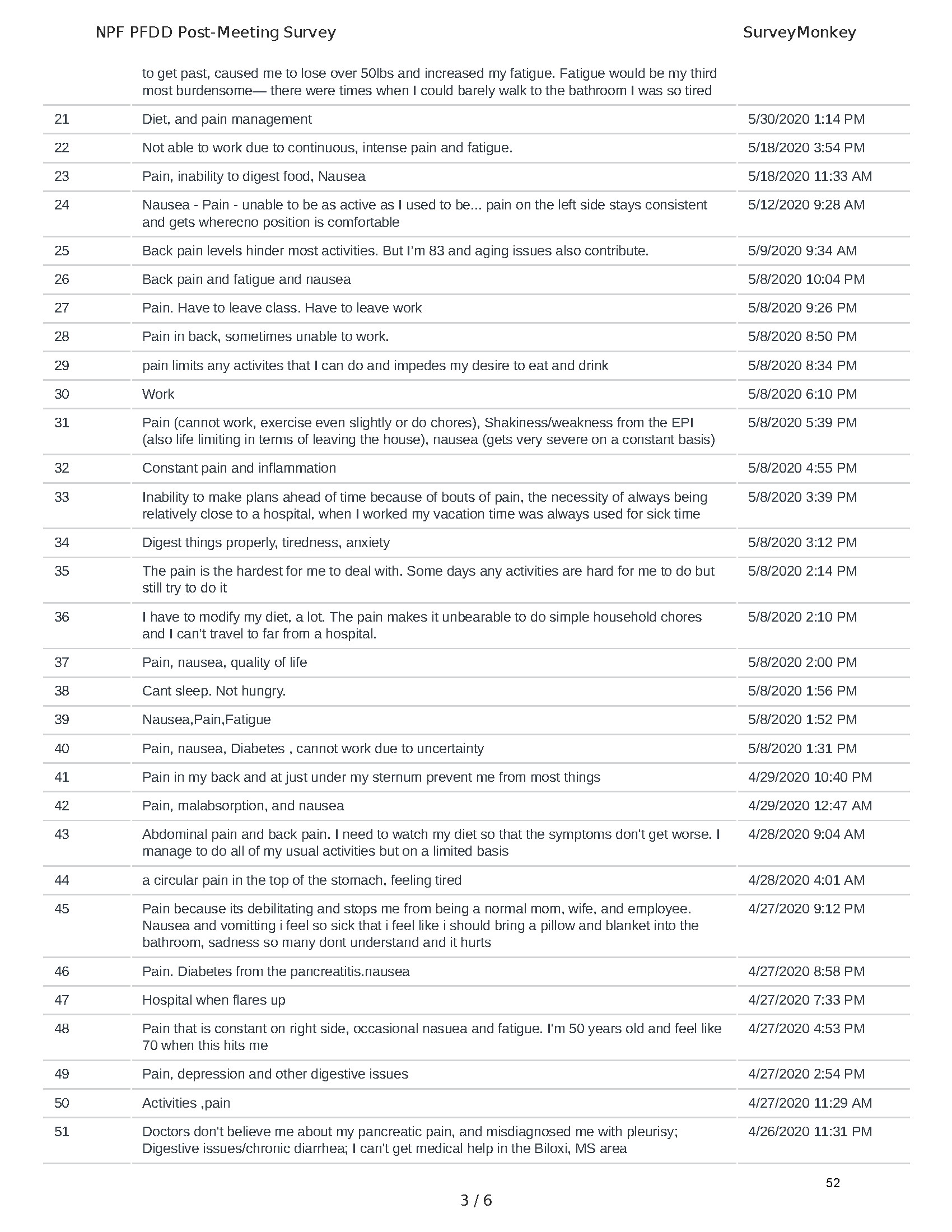
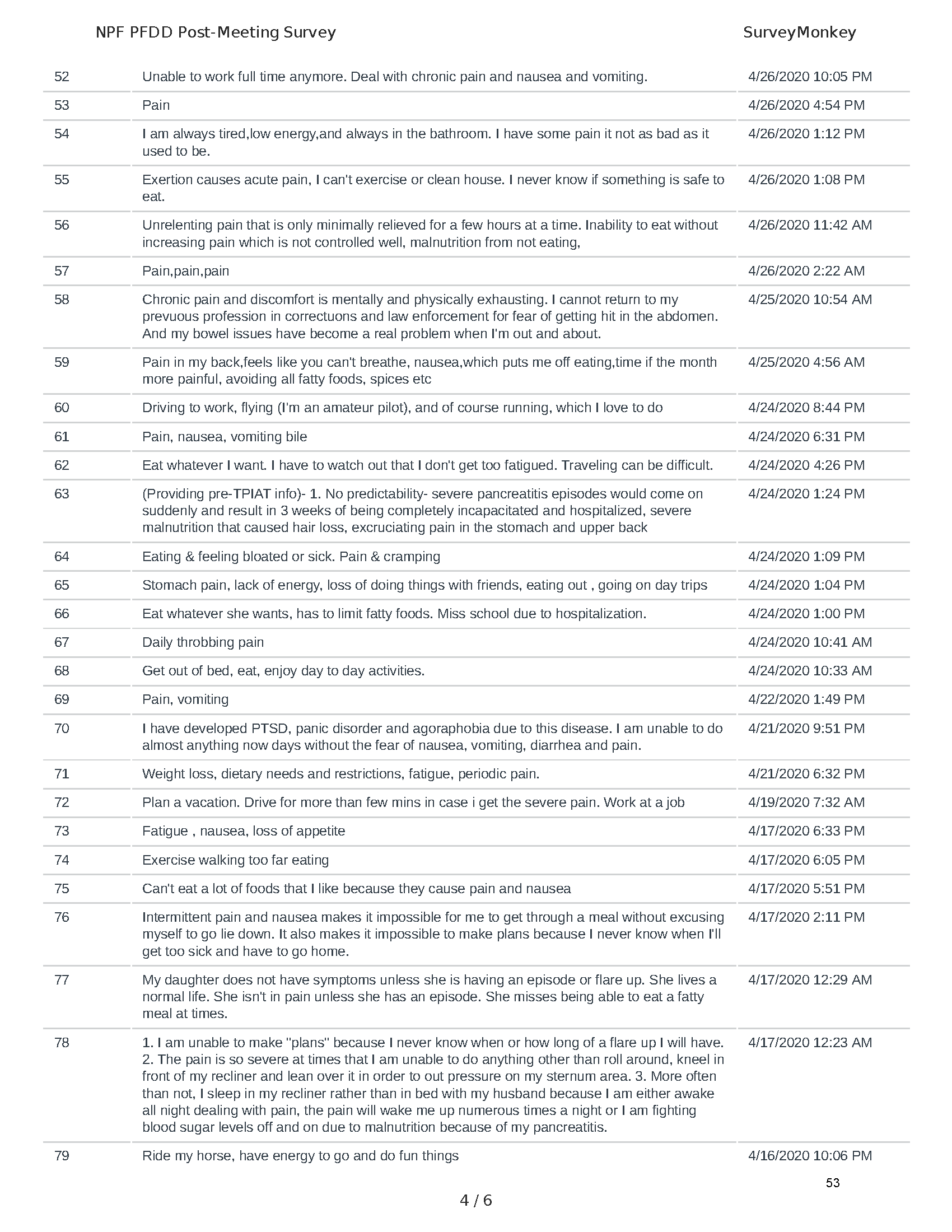
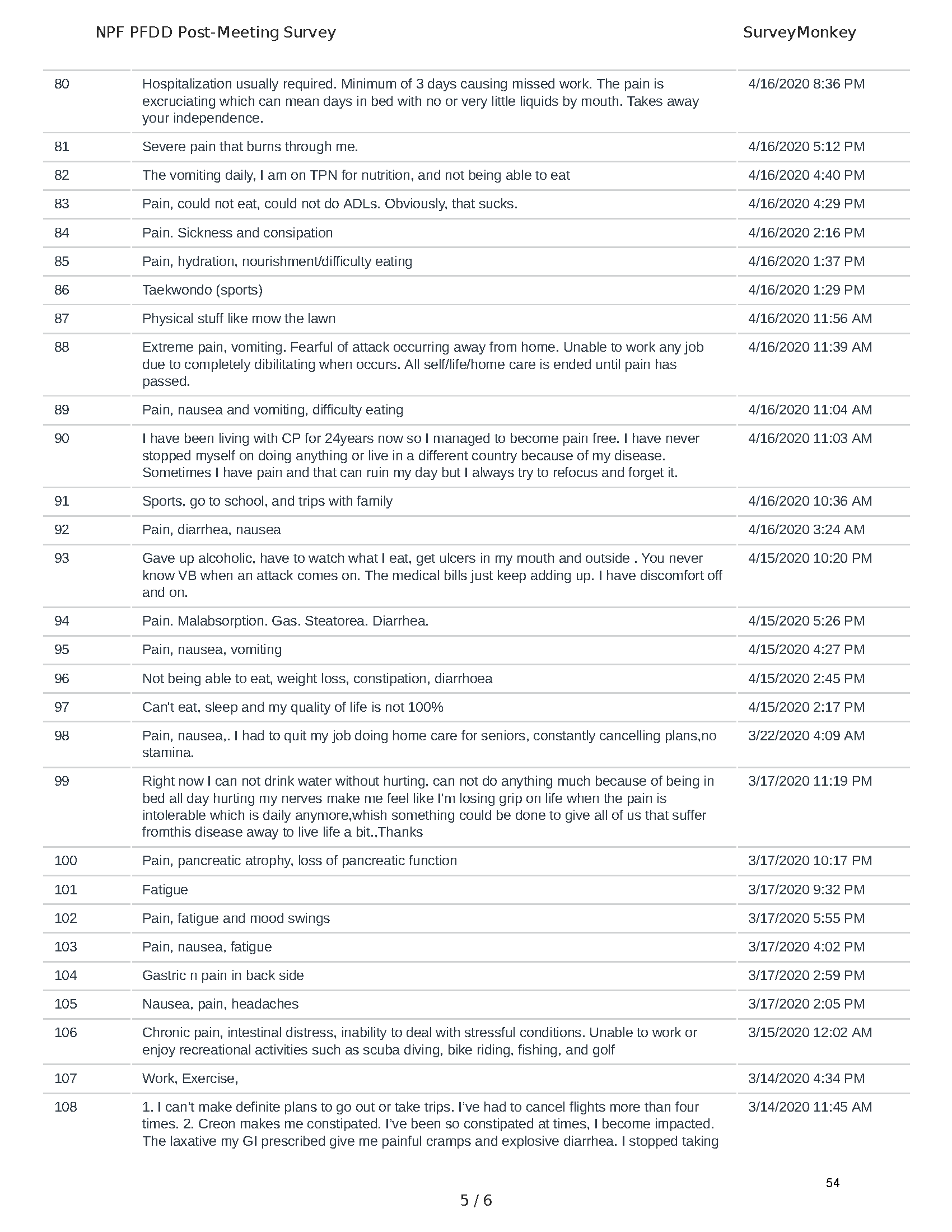
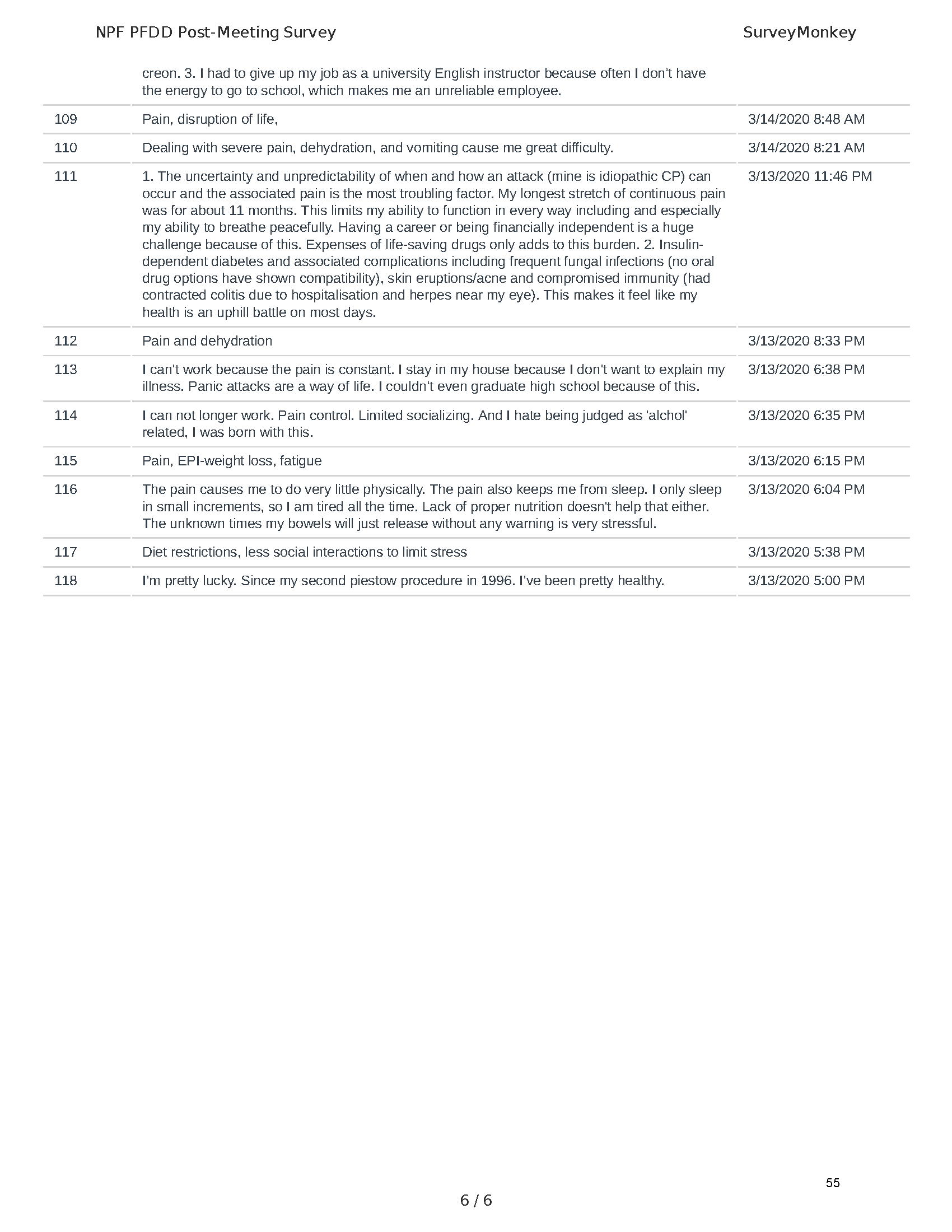
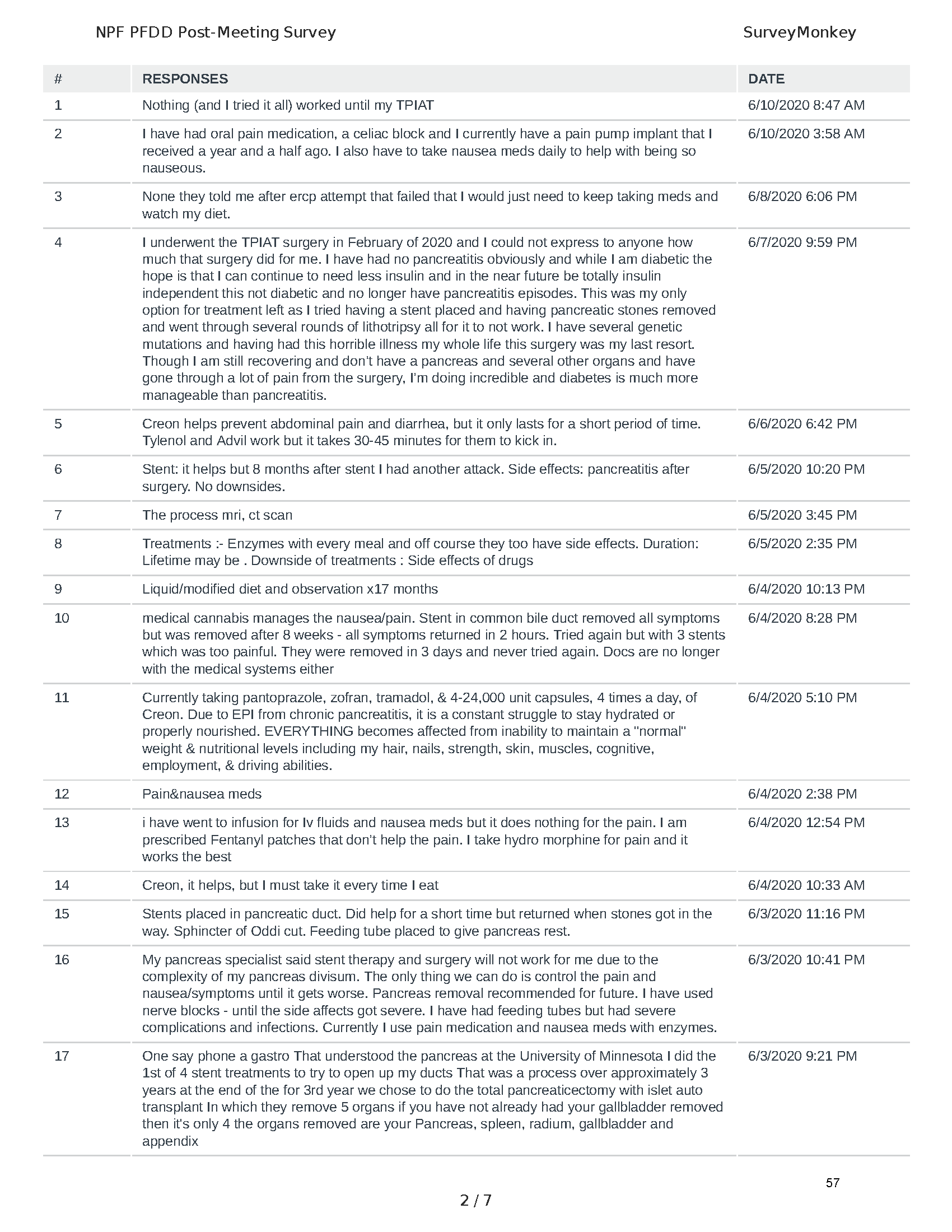
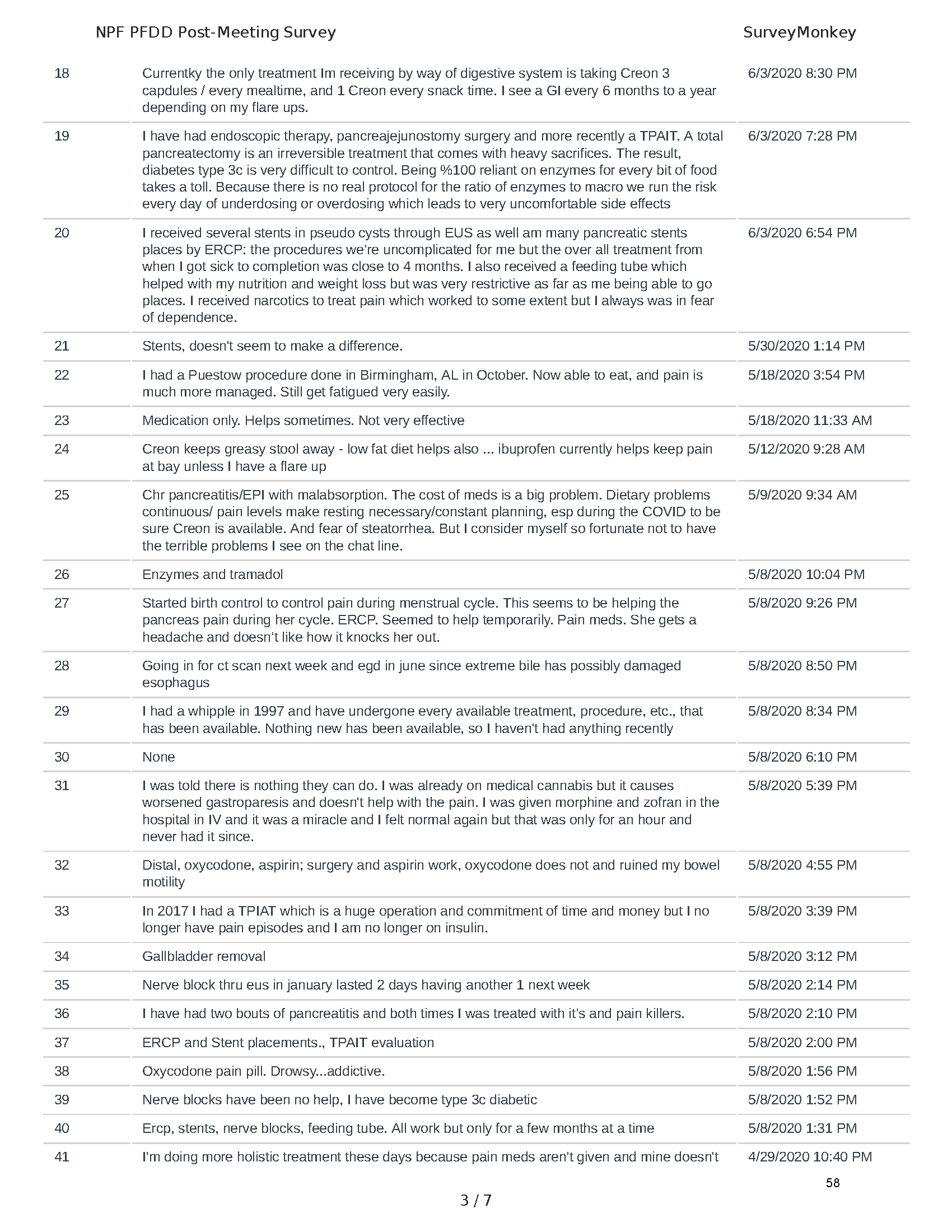
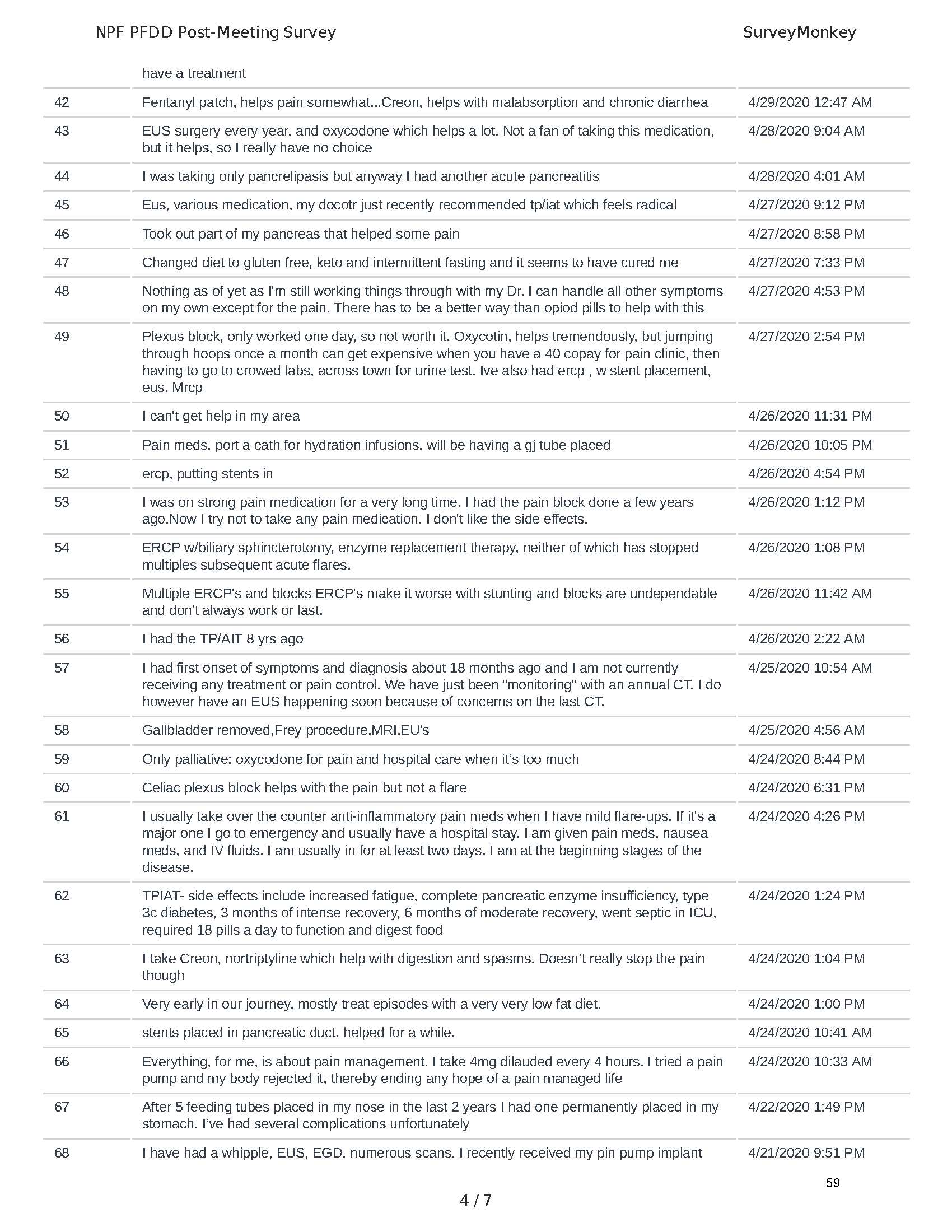
Mission: Cure was one of many patient advocacy nonprofit organizations that participated in the development of the very first Voice of the Patient Report, a months-long project led by the National Pancreas Foundation. In March, during the Patient-Focused Drug Development (PFDD) meeting, Co-Founder and pancreatitis patient Eric Golden shared his experience with pancreatitis before members of the United States Food and Drug Administration (FDA). PFDD meetings put patients’ experiences at the forefront and give them a voice in the drug development process.























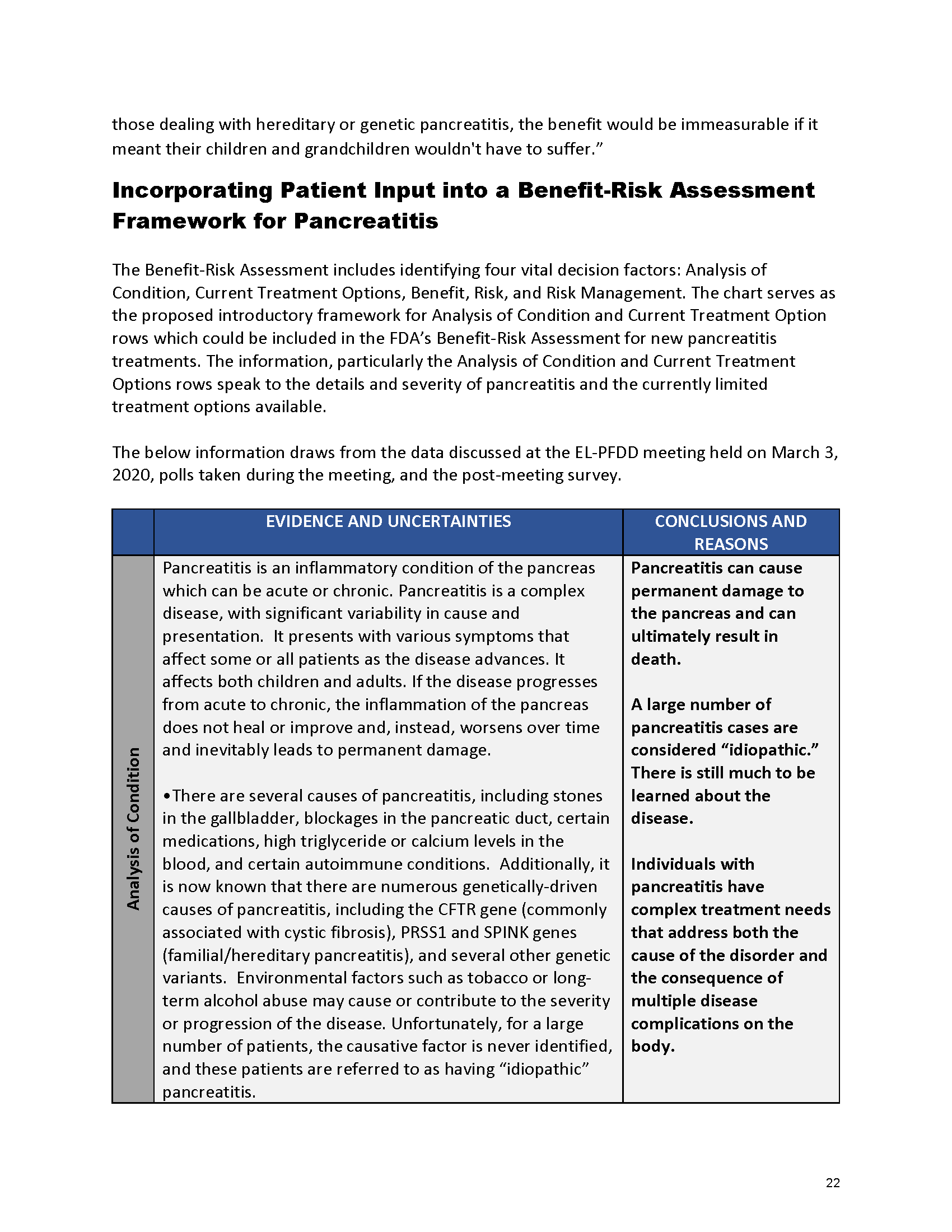
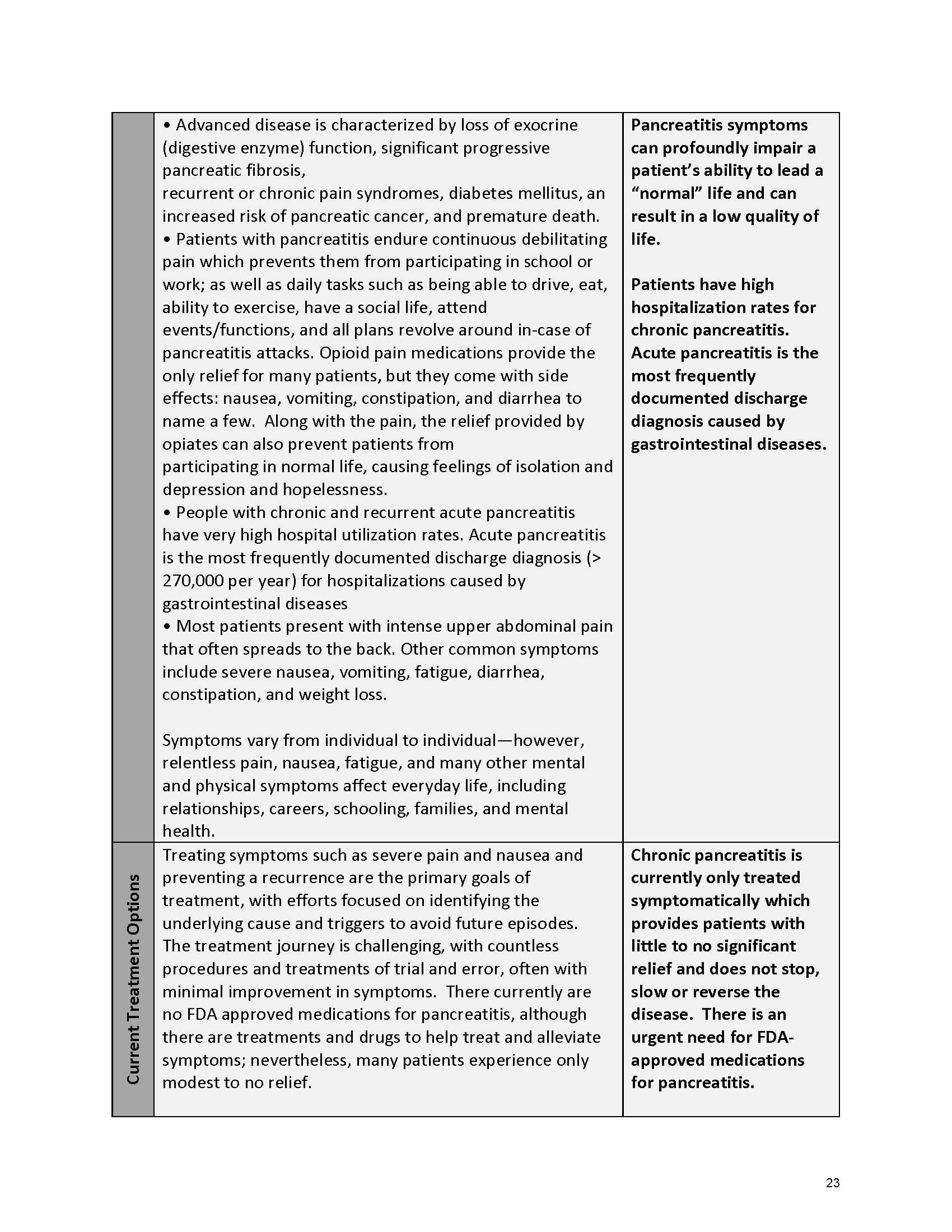
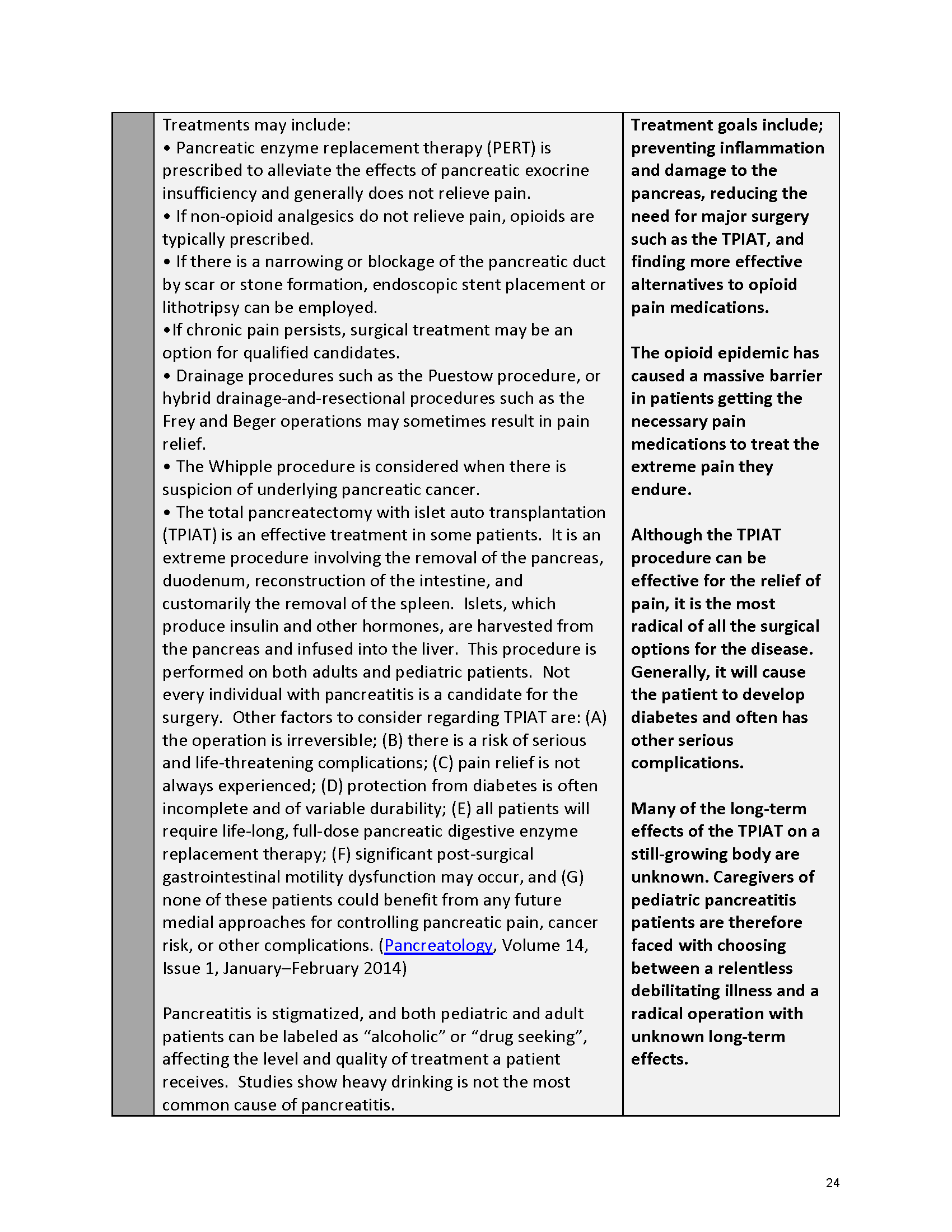
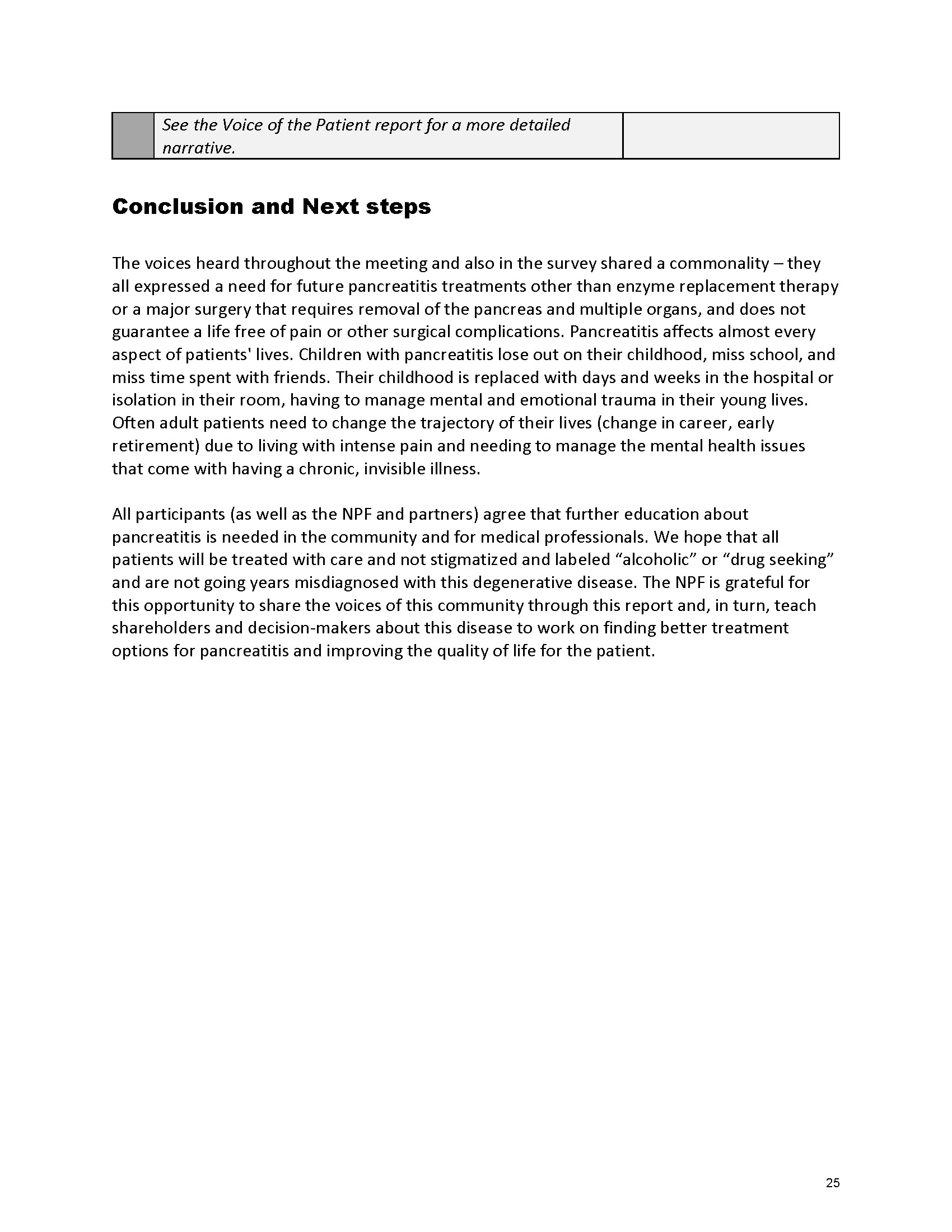












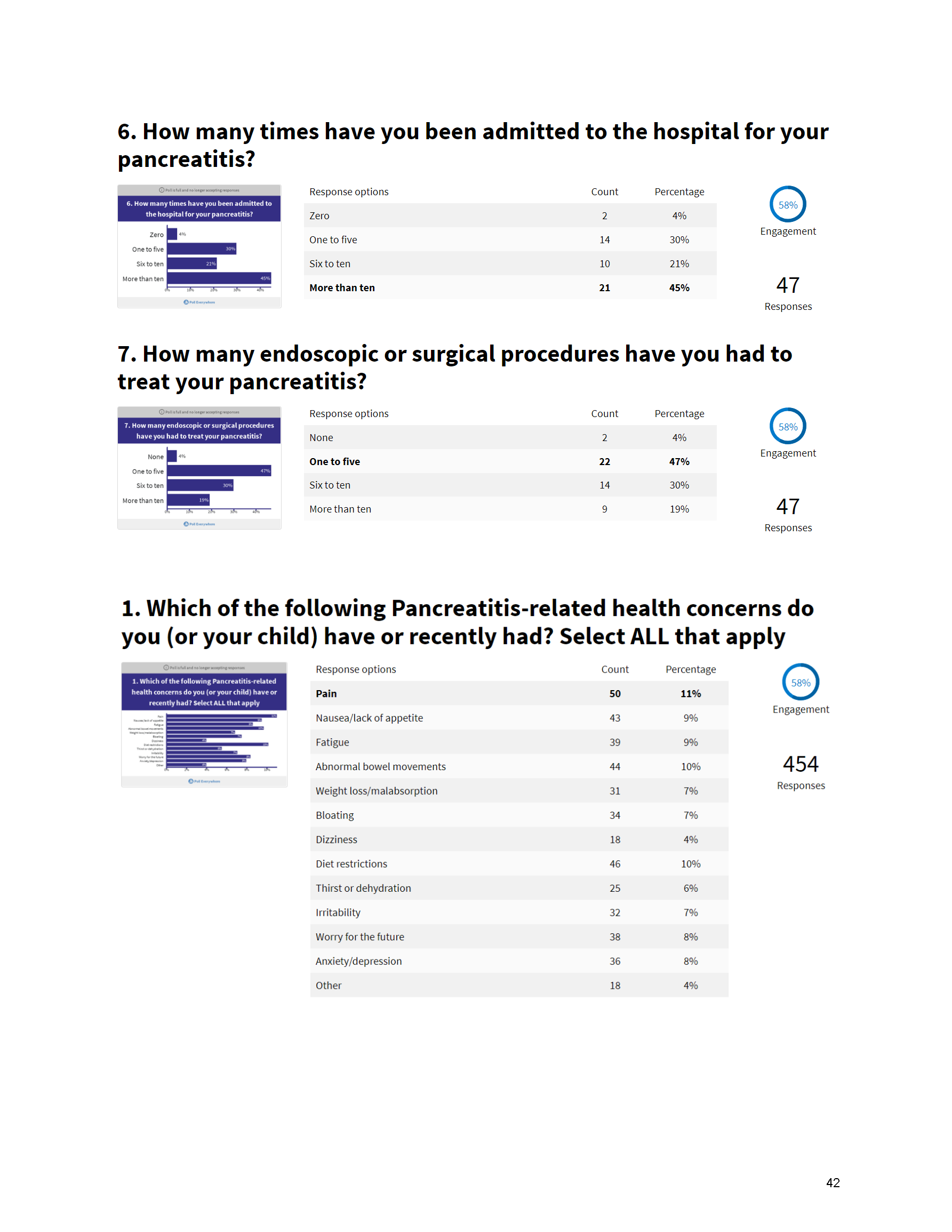
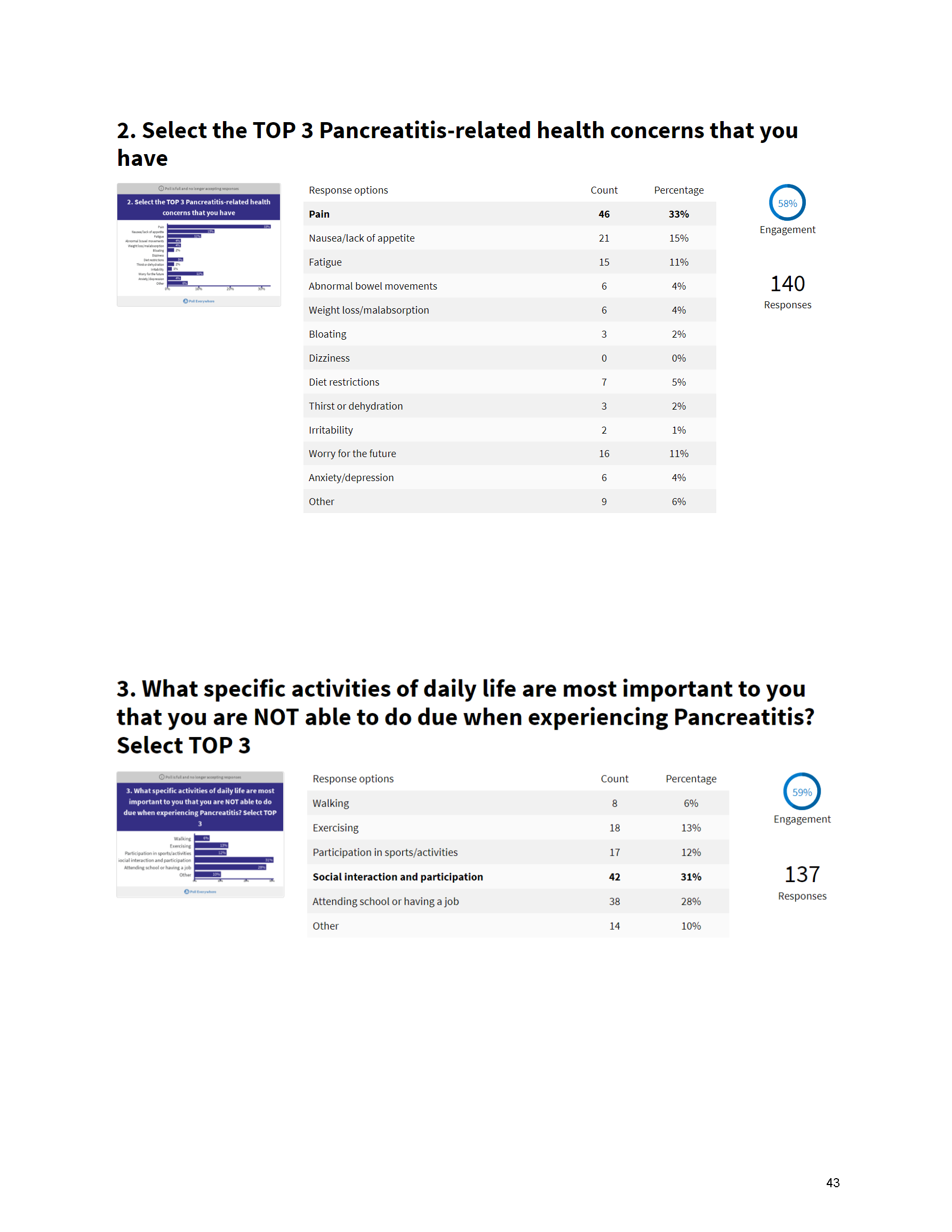
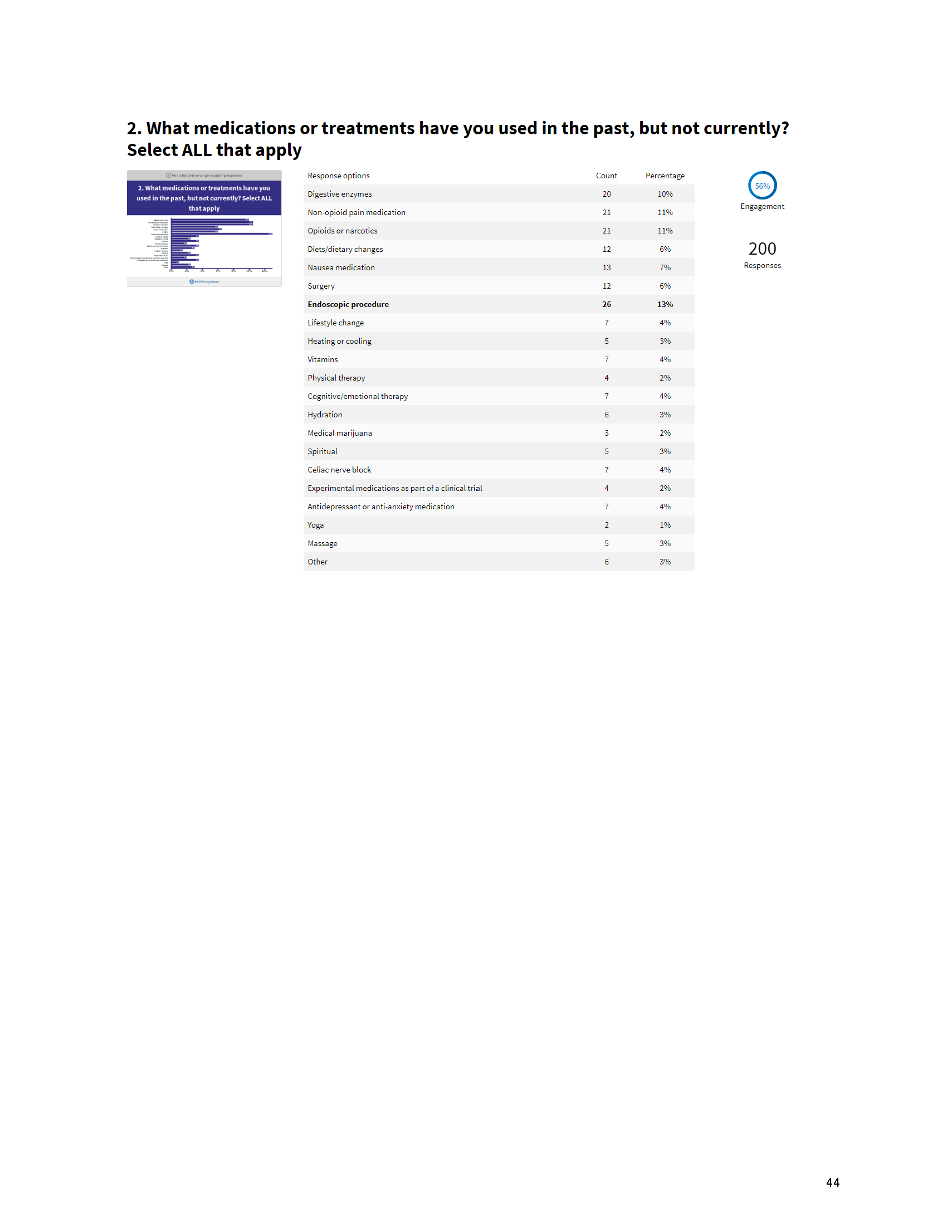
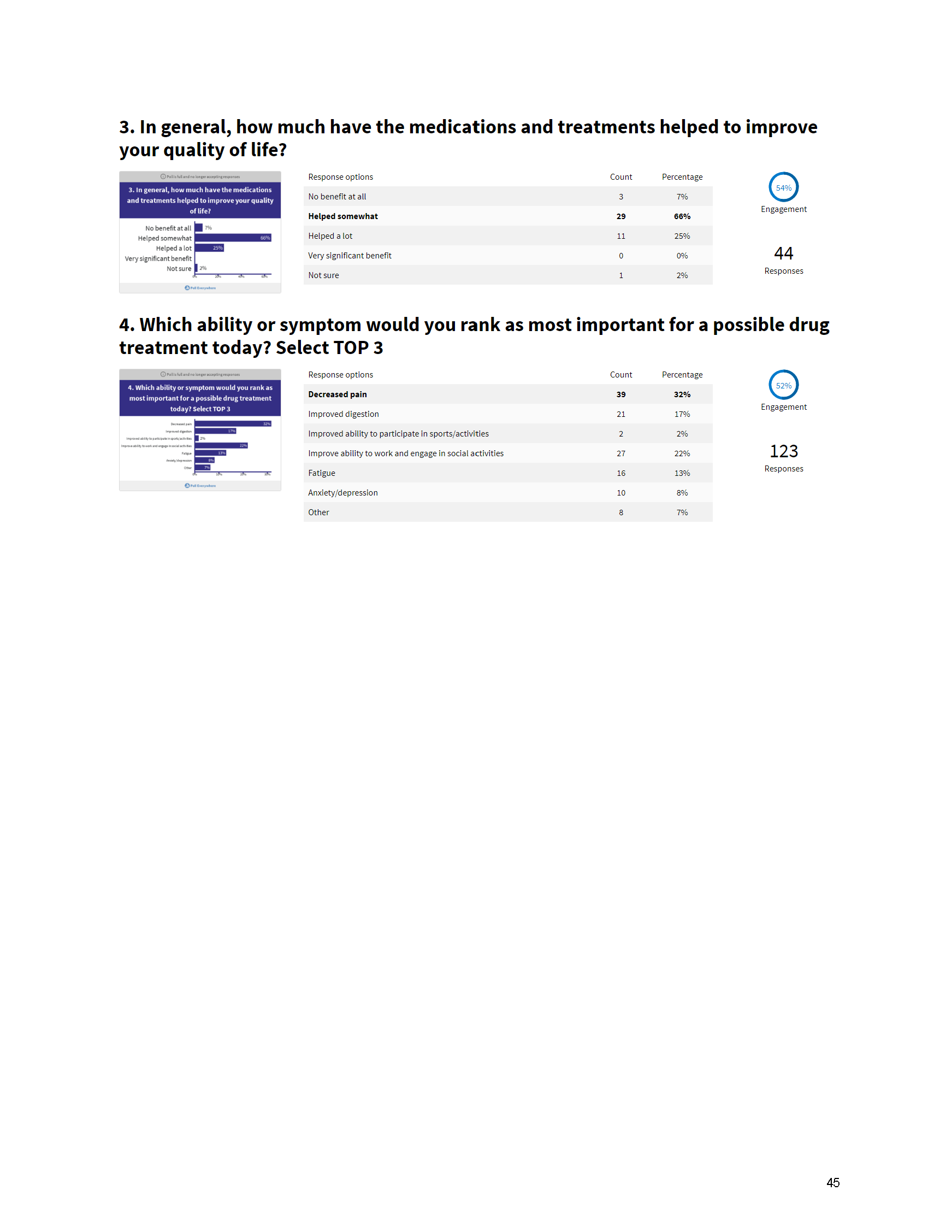



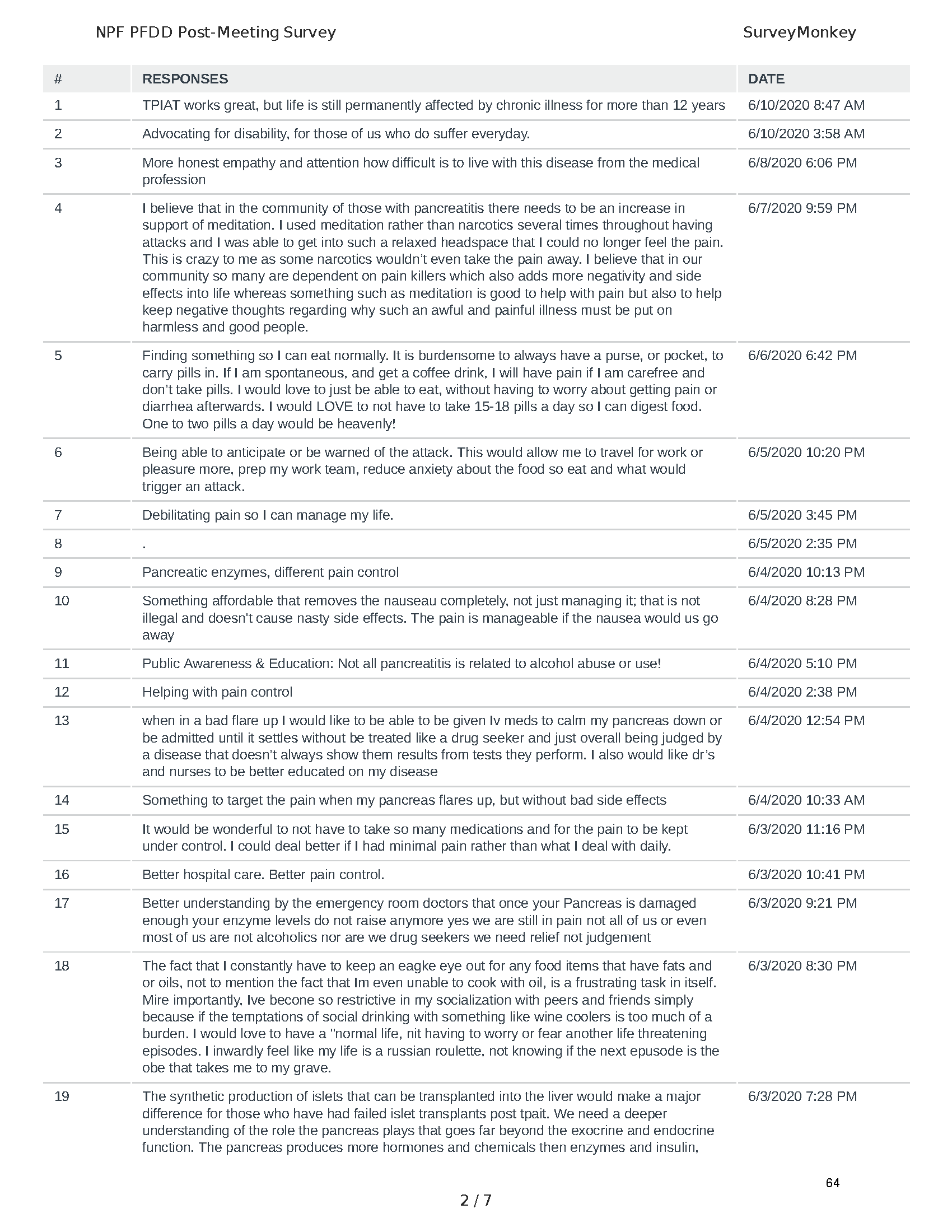








Mission: Cure’s Belief in Patient-Centered Care
At Mission: Cure, we know firsthand the importance of a care model that puts patients at the forefront. We envision high quality care and support for pancreatitis patients that provides measurable improvement in patient outcomes and experience.
The PFDD meetings are a step in this direction– a commitment on our behalf to put patients’ voices first during every step of the disease process, including drug development. From NPF’s reporting: “Members of the FDA attended the meeting to listen to patients, caretakers, and other patient representatives discuss patients’ experiences with pancreatitis, the disease burden on daily life, available management approaches, and hopes for future treatments.”
Patient-focused drug development is at the core of Mission: Cure’s innovative approach of getting to cures for pancreatitis and restructuring patient care to put patients first. Read more here about the steps Mission: Cure is taking to develop a patient-centered care model for pancreatitis.

